
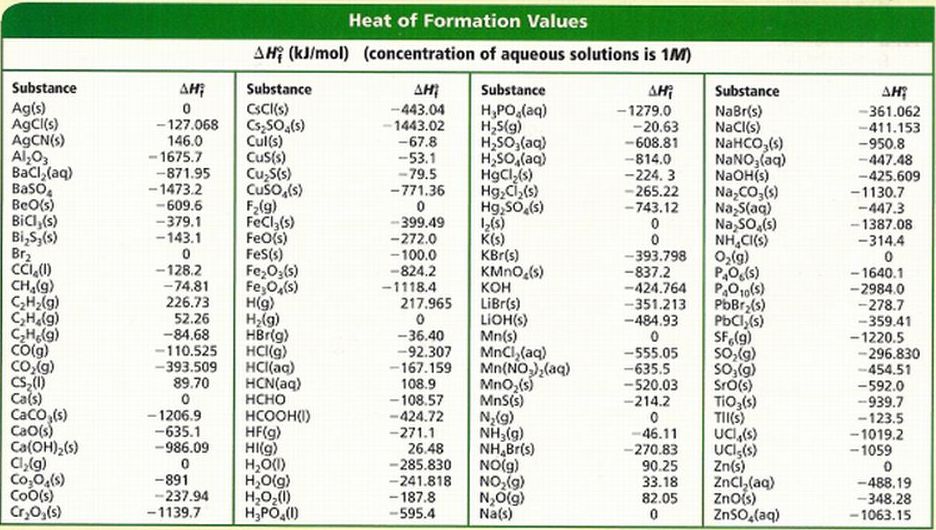
The tables four legs are constructed of cubes stacked on top of each other, all the way up to the tables top surface. A cube is the epitome of ordertheir equal X, Y, and Z dimensions make them somewhat like a Cartesian building block. The freedom in that part of the universe may increase with no change in the freedom of the rest of the universe. The Entropy Tables design revolves around the manipulation of cubes. Statistical Entropy - Mass, Energy, and Freedom The energy or the mass of a part of the universe may increase or decrease, but only if there is a corresponding decrease or increase somewhere else in the universe.Qualitatively, entropy is simply a measure how much the energy of atoms and molecules become more spread out in a process and can be defined in terms of statistical probabilities of a system or in terms of the other thermodynamic quantities. Statistical Entropy Entropy is a state function that is often erroneously referred to as the 'state of disorder' of a system.Phase Change, gas expansions, dilution, colligative properties and osmosis. Simple Entropy Changes - Examples Several Examples are given to demonstrate how the statistical definition of entropy and the 2nd law can be applied.A microstate is one of the huge number of different accessible arrangements of the molecules' motional energy* for a particular macrostate. Table 1: A Maximum Entropy Species Distribution Model to Estimate the Distribution of Bushpigs on Madagascar and Its Implications for African Swine Fever. Instead, they are two very different ways of looking at a system. In particular, we search for ordering that minimizes entropy of residuals of predictive coding applied on the ordered data table. Microstates Dictionaries define “macro” as large and “micro” as very small but a macrostate and a microstate in thermodynamics aren't just definitions of big and little sizes of chemical systems.“Disorder” was the consequence, to Boltzmann, of an initial “order” not - as is obvious today - of what can only be called a “prior, lesser but still humanly-unimaginable, large number of accessible microstate it was his surprisingly simplistic conclusion: if the final state is random, the initial system must have been the opposite, i.e., ordered. ‘Disorder’ in Thermodynamic Entropy Boltzmann’s sense of “increased randomness” as a criterion of the final equilibrium state for a system compared to initial conditions was not wrong.If a “Pillet style” exists, it is in his ability to crystallize, within a project, the excitement of a proposition.\) Whether it be as design director for renowned fashion brands, or in longterm collaborations with first class furniture brands, his considered interpretations are a testo high-voltage chic, distinguished both by its precision and rigor. Architecture objects, furniture, artistic direction, his signature is invariably associated with the best furniture companies and with the finest luxury brands: Trussardi, Lacoste, Moët et Chandon, Veuve Clicquot, Lancome, Shiseido, l’Oréal, JC Jitrois, Catherine Malandrino, Lancel, Le Tannneur International… The scope and the variety of his projects share a common attitude, independent of scale. He has won international acclaim for the spectrum and quality of his creations. Pillet’s perfect command of sensuality and refinement has made him one of the rare French designers who have gained global recognition designing hotels, boutiques and directing artistic projects in Europe, USA, and Japan. Reaction thermochemistry data: reactions 1 to 50, reactions 51 to 100, reactions 101 to 150, reactions 151 to 200, reactions 201 to 250, reactions 251 to 300, reactions 301 to 350, reactions 351 to 400, reactions 401 to 450, reactions 451 to 500, reactions 501 to 550, reactions 551 to 600, reactions 601 to 650, reactions 651 to 700. Lucidity of expression and the search for simplicity are the key principles: in the work of Christophe Pillet the elegance is optimized.
During his career he has published many articles on the most important magazines of the world. Pillet was elected as “Créateur de l’année 1994”. Since 1993, he manages his own design agency. the entropy of a pure substance at 298 K and 1 atm pressure).
Entropy table series#
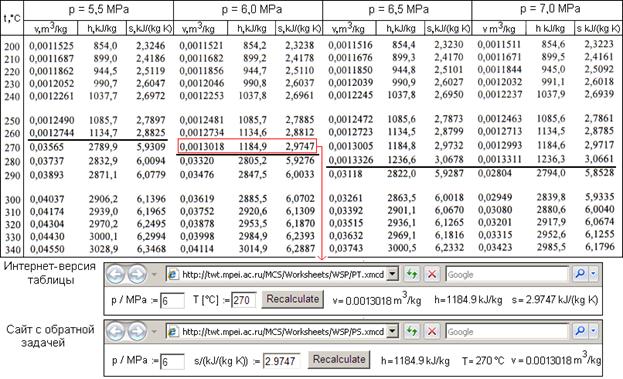
Standard molar entropies are listed for a reference temperature (like 298 K) and 1 atm pressure (i.e. Assuming the dataframe is containing the value counts for each name, you can directly feed a Series of counts to : from scipy. He worked with Martine Bedin in Milano from 1986 to 1988 and cooperated with Philippe Starck, in Paris, from 1988 to 1993. The entropy of a substance has an absolute value of 0 entropy at 0 K. Christophe Pillet graduated from the Decorative Arts School in Nice in 1985, Master of Domus Academy Milano, in 1986. A new look at entropy and the life table, Demography, Springer Population Association of America (PAA), vol.


 0 kommentar(er)
0 kommentar(er)
